The problem of residual elements in steel is one of the important problems in metallurgical industry. In the process of steelmaking, the raw materials (including hot metal, scrap and ferroalloy) will bring a lot of impurity elements into the furnace. Some of the impurity elements can be removed, but some impurity elements will remain in the steel, which are collectively referred to as residual elements.
These residual elements are one of the main factors causing instability of steel quality. Some residual elements are easy to segregate, even if their content is very low, it will have a strong negative effect on steel properties.
For example, the residual titanium in bearing steel is a typical case. Ti is easy to react with n to produce high hardness inclusions, which greatly affects the service life of bearing steel.
1. Classification of residual elements
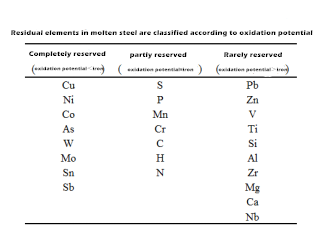
Some known residual elements in steel can be divided into three categories according to their oxidation potential, as shown in the table below. They are completely retained, partially retained and rarely retained in the steelmaking process.

In the table above, the oxidation potential of the first group of elements is lower than that of iron, and they do not participate in the oxidation reaction during steelmaking, and almost all of them accumulate in steel products. The oxidation potential of the second kind of residual elements is close to that of iron. In the process of steelmaking, only a part of them are removed by oxidation, and the degree of removal is related to the characteristics of the elements themselves. The oxidation potential of the third kind of elements is higher than that of iron. In the process of molten steel blowing, they are first oxidized into the slag to be removed, and only a small part of them enter into the product. Therefore, there are only 15 elements in the first and second categories of steel. Among them, 8 elements are fully reserved elements, and 7 elements are partially reserved elements.
2. Source of residual elements in steel
China is a country with a large number of intergrowth iron ores, including V, Ti, P, as, Sn, Sb, re (rare earth elements), etc., which are brought into steel during smelting. In addition to the residual elements brought into molten iron by primary iron ore, the largest source of residual elements in molten steel is scrap steel
(1) Alloy steel in scrap. At present, there is no economic and effective technology to separate alloy steel and ordinary carbon steel, but there are many kinds of alloy elements in some medium and high alloy steel. In the recycling of steel, these alloy elements will enter the steel as residual elements;
(2) Surface coating or coating in scrap steel. Among them, tin plate is the most problematic one, which enters the scrap recycling as a can box, and other coatings include copper, nickel and chromium; galvanized sheet is also widely used, but zinc can be basically removed in steel-making without consideration; (3) non-ferrous metals wrapped in scrap raw materials. The most important is scrap steel, which contains some micro motors, the main impurity is copper. On the market, the most residual element content is copper, which is mainly from automobile scrap into the steelmaking furnace. It is estimated that the average copper content in steel scrap is about 0.3%, which depends on the source and proportion of alloy steel. The residual sb and as in the steel mainly come from the primary iron ore. when the scrap containing these impurities is recycled, they can be diluted, but the residual amount will gradually accumulate in the steel. The content of H and N in steel mainly comes from the atmosphere in the steel-making workshop, and its content mainly depends on different steel composition and steelmaking process.
3. Segregation of residual elements in steel

Many residual elements exist and play a role in steel in the form of segregation. Most of the residual elements have strong segregation ability in steel; the segregation process of these elements can occur not only in the solidification process of liquid steel, but also in the subsequent solid phase transformation, but it needs a long diffusion time. The main segregation elements in the riser of ingot are s, P and C, followed by sb, N, as, h and Sn. The hardness of this part of material is higher than that of other parts of ingot after segregation. Compared with the solidification segregation, the residual elements will produce grain boundary segregation during solid phase transformation or heating. For example, the second temper brittleness of steel is mainly caused by grain boundary segregation of P, Sn, as and sb.
4. The function of residual elements
① Eight fully retained elements
Ni, Co, W and Mo can improve the hardenability of steel, which are beneficial elements. On the one hand, Cu can cause copper embrittlement during high temperature hot working, but on the other hand, it can improve the atmospheric corrosion resistance of steel; the residual elements Sn, as and Sb are harmful elements, which not only strengthen copper brittleness in steel, but also lead to the second temper brittleness of alloy steel; SN is one of the most harmful residual elements in steel, which can greatly reduce the high temperature mechanical properties of steel and alloy.
② 7 partial retention elements
C. Mn, s and P are the conventional control elements; Cr can improve the oxidation resistance of steel, increase the corrosion resistance and hardenability of steel, but also increase the temper brittleness of steel; n is beneficial to control the grain size of austenite, but also can cause the strain aging of steel; h in steel is a kind of harmful and harmful element, which will lead to white spots and cracks in high strength low alloy steel.