Phase Composition of WC-Co Cemented Carbides
Figure 1 shows the vertical section of the W-Co-C ternary phase diagram along the Co-WC line. Taking a WC-60%Co alloy as an example:
Before liquid phase formation, the solubility of WC in Co increases with temperature.
At the eutectic temperature (~1340°C), a liquid phase with eutectic composition begins to form in the sintered body.
During sintering at 1400°C and subsequent holding, the sintered body consists of a liquid phase and residual WC solid phase.
Upon cooling, WC first precipitates from the liquid phase. Below the eutectic temperature, the WC-based carbides forms a two-phase structure of WC + γ.
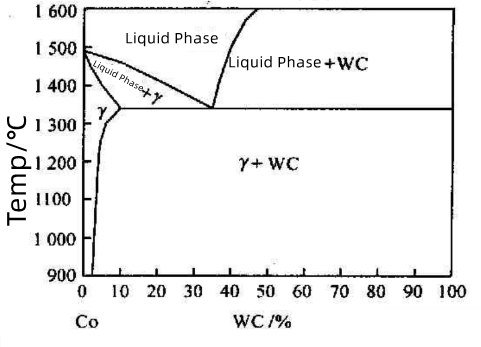
Figure 1: Vertical Section of the W-Co-C Ternary Phase Diagram Along the Co-WC Line
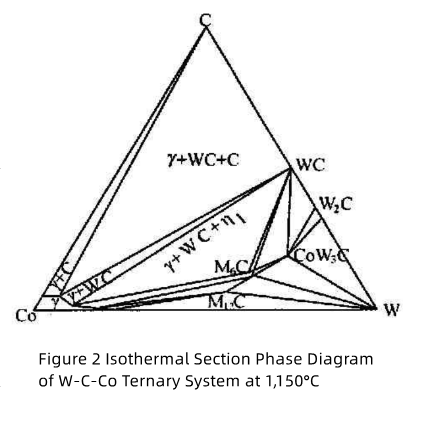
In actual production, the composition of sintered bodies often deviates from the vertical section of the Co-WC line. Consequently, the alloy is not simply composed of γ+WC two phases. As shown in Figure 2 , the carbon-rich side of the γ+WC two-phase region borders the γ+WC+C three-phase region and the γ+C two-phase region, while the carbon-deficient side borders the γ+WC+η three-phase region. Only when the carbon content of the sintered body varies strictly within the γ+WC two-phase region can the WC-based carbide avoid the formation of a third phase. Otherwise, it may lead to carbon inclusions or the formation of carbon-deficient η phase.
Since the strength of the alloy is closely related to the structure and composition of the γ phase, while the presence of η phase may degrade toughness, extensive research has been conducted on the γ and η phases, as well as phase transformation processes, in an effort to control the phase composition of WC-Co alloys and improve their overall performance.
γ Phase Composition and Phase Transformation in WC-based carbides
As shown in Figure 2, the composition of the γ phase depends on the carbon content of the alloy, while its tungsten content increases with decreasing carbon content. When the alloy’s carbon content lies at the boundary between the γ+WC two-phase region and the γ+WC+η three-phase region, the γ phase exhibits the highest tungsten concentration. Conversely, when free carbon is present and the carbon content aligns precisely with the Co-WC cross-section (i.e., the theoretical carbon content of 6–12 wt.%), the γ phase contains the lowest tungsten concentration.
The tungsten concentration in the γ phase is also influenced by the cooling rate: slower cooling results in lower tungsten content, while rapid cooling leads to higher tungsten retention. This occurs because faster cooling suppresses the diffusion-driven precipitation of tungsten from the γ phase, locking in a non-equilibrium concentration. Additionally, higher sintering temperatures increase the tungsten solubility in the liquid phase, thereby raising the tungsten content in the γ phase at a given cooling rate. However, under sufficiently slow cooling, thermodynamic equilibrium dictates that the γ phase composition becomes independent of the sintering temperature.
In WC-Co cemented carbides, the γ phase is a cobalt-based solid solution of W and C. It exists either as discrete γ grains separated by grain boundaries or as isolated γ domains unevenly distributed within the matrix. Both γ grains and domains typically exhibit equiaxed or near-equiaxed morphologies. Notably, the volume fraction of γ domains increases with higher cobalt content in the WC-based carbide.
Factors Influencing γ Phase Transformation in WC-based carbides
Effect of Internal Stresses
The mismatch in thermal expansion coefficients between WC phase (384×10??/°C) and γ phase (1.25×10??/°C) generates microstructural stresses during cooling (tensile in γ phase, compressive in WC phase).
Increased cooling rate or quenching suppresses W diffusion precipitation in γ phase, elevating W concentration in room-temperature γ phase while reducing hcp γ phase content.
Cryogenic treatment (below Ms point) induces W supersaturation in γ phase, enlarging the free energy difference between fcc and hcp γ phases. Concurrently, enhanced internal stresses promote Ms transformation, markedly increasing hcp γ phase fraction—particularly pronounced in low-Co alloys.
Impact of Cobalt Content
In low-Co alloys (e.g., WC-8Co), thin γ phase layers (<0.3 μm) facilitate W diffusion to WC grains, lowering W concentration in γ phase. This raises the Ms point, favoring hcp γ phase formation during cooling and yielding higher room-temperature hcp γ phase content.
η Phase in WC-based carbides
Formation Mechanism and Morphology of η Phase
Due to the narrow carbon content range in the WC-γ two-phase region (Fig. 2), carbon deficiency in raw materials or sintering decarburization often leads to η phase formation (e.g., M?C-type Co?W?C, Co?W?C, and M??C-type Co?W?C). Among these, Co?W?C is most common.
Formation process
Heterogeneous nucleation: γ phase nucleates along WC-γ interfaces using WC grain surfaces as nucleation sites, facilitated by slow W diffusion from WC to γ phase and high W concentration at phase boundaries. γ phase tends to fill surface defects (high-energy sites) of WC grains.
Carbon loss and η phase precipitation
Rapid C diffusion in γ phase causes C depletion when WC dissolves, resulting in W/C ratio imbalance (room temperature [W]/[C]≈284).
During sintering (1350-1500°C), excessive C loss leads to W-rich γ phase, precipitating carbon-deficient η phase (intermediate phases like Co?W and Co?W?C form first, transforming to η phase at high temperatures).
Phase equilibrium and morphology
η phase growth consumes W and C, driving WC dissolution until equilibrium is reached.
η phase morphology is influenced by γ liquid phase flow (e.g., cross-shaped single crystals).
Key point: Carbon imbalance is the primary cause of η phase formation, with γ phase nucleation dependent on WC interfaces and high-temperature C loss driving η phase precipitation.
Factors Influencing η Phase Formation
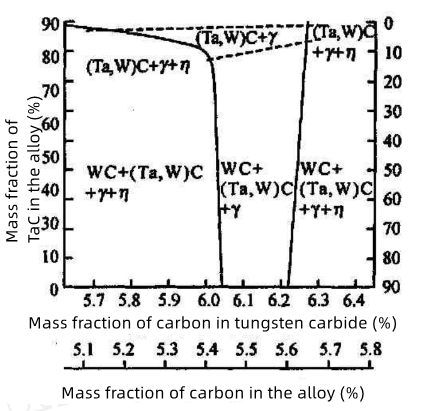
Carbon content is critically important for η phase formation. In the WC+γ+η three-phase region:
Higher carbon content maintains W and C concentrations in γ phase closer to equilibrium, hindering η phase nucleation.
Mild carbon deficiency: η phase growth relies on dissolution of WC microcrystals in γ interlayers, resulting in η phases enveloping undissolved WC grains with regular geometries.
Severe carbon deficiency: Significant deviation from equilibrium W/C ratio in γ phase promotes extensive WC dissolution, leading to dispersed particulate η phase distribution.
Cobalt content effects
High-Co alloys contain more γ phase with better fluidity, facilitating W and C diffusion. While η phase nucleation is difficult, growth is easier, forming coarse, clustered grains.
WC grain size effects
Coarser WC grains promote η phase nucleation but slow growth, resulting in dispersed particulate phases.
Sintering process effects
Faster cooling reduces dwell time at η phase critical temperature, suppressing η phase formation.
Higher sintering temperatures increase γ liquid phase quantity, favoring coarse η phase grains, but excessive temperatures may keep γ liquid away from η phase boundaries, inhibiting η phase growth.
Conclusions
A comprehensive understanding of the phase transformation processes during the sintering of WC-based carbides is crucial for optimizing production processes, controlling phase composition and microstructure in the alloys, thereby creating favorable conditions for manufacturing high-performance WC cemented carbides.
Schreibe einen Kommentar
Deine E-Mail-Adresse wird nicht ver?ffentlicht.